Drug Discovery and Market Factors within the Atypical HUS Arena

E: info@aHUSallianceAction.org Atypical HUS – Global Rare Disease Advocacy
The aHUS Alliance is an international group of people and organizations interested in the advancement of research and advocacy that impacts those living with the rare disease atypical hemolytic uremic syndrome (atypical HUS or aHUS). All rare disease populations have difficulty finding information and resources, but the atypical HUS community faces additional difficulties in part due to: its rarity and small patient population, variations in different spellings and abbreviations of the disease name, and variety of disease classification among researchers and clinicians1. Whether it’s listed as “atypical haemolytic uraemic syndrome”, the abbreviation aHUS (which switches to SHUa in French and Spanish) or found listed under different terms such as ‘familial HUS’, research regarding atypical HUS is found not only in nephrology journals but also scattered throughout hematology, immunology, heme-onc, and immunology medical literature as well. Important advancements and new knowledge may be listed under categories such as inflammation or auto-immune disease research. The aHUS Alliance regularly reports on international meetings with agenda items related to the atypical HUS space, to include medical conferences and global research summits. We frequently cross-search content, since new information or research about atypical HUS might appear under headings such as ‘complement mediated’ (e.g. complement-mediated HUS, CM-HUS) or ‘thrombotic microangiopathy’ (TMA), without any actual mention of aHUS as a keyword in any of its various terms, spellings, or names.
It should be no surprise that an international patient advocacy group such as the aHUS Alliance seeks a clear view of upcoming clinical trials and specifics about what new treatment options might be approaching on the horizon. Offered here is an outline of the complex landscape of what aHUS research and development looks like from our perspective, using a unique and authentic patient advocacy voice to examine factors within the current landscape of aHUS drug development. Presented in a context which additionally may serve to highlight current issues facing aHUS families and note avenues of value for all stakeholders to consider, the aHUS Alliance presents this basic yet holistic view of aspects related to expansion of aHUS therapeutic drug pipeline during 2018. (For detailed aHUS medical information, click to read NIH GeneReviews®)
Trends in 2018 aHUS Drug Discovery
Atypical HUS patients and their caregivers want a safe and effective drug with relative ease of administration and at a reasonable price point. Orphan drug research is expanding, and aHUS patient organizations around the world are interested in research and potential new drug therapies for people living with atypical HUS. The fluid nature of drug discovery remains a challenge for all stakeholders, to include the aHUS Alliance and atypical HUS patients, families, and advocacy organizations around the world. While other players change within the aHUS arena, people directly affected by the disease remain constant. One current trend in atypical HUS investigational drug R&D seems to be toward formulation advancements that offer longer dosing intervals, such as in Akari Therapeutics’ exanded pipeline with three versions of Coversin®. Although Achillion Pharmaceuticals’ focus currently is directed more toward a therapeutic drug for PNH patients, it’s interesting to see that ACH-4471 also is listed on corporate materials to include an alternate formulation for an extended release tablet (ACH-4471XR). Plant-based medicine has deep and historic roots, continuing today through innovative and promising biopharmaceutical research such as Moss-FH, under development at the Reski Labs and Greenovation. Clinical trials for atypical HUS will prove to be ‘Ones to Watch’ in 2018, with listings on Clinicaltrials.gov that currently include various trials for Alexion’s eculizumab and AXLN1210, for Alnylam’s Cemdisiran (NCT03303313: formerly known as ALN-CC5), for ChemoCentryx’ Avacopan (NCT02464891: formerly known as CCX168), and for Omeros’ OMS721 (NCT03205995). Atypical HUS is considered one type of thrombotic microangiopathy (TMA is not a disease, but a pattern of injury) so information regarding TMA clinical trials bears watching as well. In addition to delving deeper into extended dosing, we’re seeing more options noting “SC” to provides an indication that drug delivery is subcutaneous and meaning ‘beneath the skin’ (which most patients would identify as a ‘shot’ or injection). Cooperative arrangements among companies seem to be offering positive steps forward for drug discovery pipelines which may advance potential aHUS therapies. (See Table 1, aHUS Alliance 2018 pharma overview)
Corporate acquisitions and partnerships. This trend to broaden drug R & D options through technology interests us on a variety of levels, and this past year has seen increased activity regarding corporate acquisitions and partnerships. The aHUS Alliance is aware that new knowledge and research in the fields of rare blood disorders, complement mediated diseases, or thrombotic microangiopathy syndromes may hold potential to advance aHUS research and treatment as well. (See Table 2 for diseases which may hold potential for knowledge cross-over to aHUS research). We’ve previously expressed interest True North Therapeutics, designated earlier by the aHUS Alliance within our ‘Ones to Watch’ list due to its status as a rare disease biotechnology company with efforts and interests in complement research. A June 2017 press release by Bioverativ noted their acquisition of True North Therapeutics, to deepen and develop this aspect of Bioverativ’s innovation in rare blood diseases. Less than a year later, Sanofi’s corporate release of 9 March 2018 stated that it acquired Bioverativ to strengthen their position in rare blood disorders. Collaboration of pharma with biotech companies may allow specialized formulations of pipeline products, such as Alexion Pharmaceuticals’ announcement of its partnership with Halozyme to explore options new drug delivery options related to its standard drug delivery of eculizumab (IV infusion). According to that press release Alexion Pharmaceutical’s use of ENHANZE® technology may provide potential for C5 complement inhibition delivered by injection, with the possibility of a dosing interval for ALXN1210SC perhaps limited to only once or twice a month. New trends in extended dosing intervals or potential for future aHUS drugs to be delivered orally (pill form) or injection (SC, shots) are likely to have a large impact on patients, their families, and healthcare costs. Various socio-economic factors are affected by the method of drug delivery, since IV drugs require different accommodations which might include lost time at work or school, distance from an infusion center, impact on the patient’s or family lifestyle.
Drug type and delivery method. Drug type and methods of drug delivery are important for a variety of cost-related reasons. Costs for creating a chemical molecule tend to be much lower, and it’s relatively simple to manufacture an identical copy in a laboratory thus paving the way for a generic version at some future point. Biological drugs are just what the term implies, drugs that start from living cell cultures which take both careful conditions and time to grow. Making a copy of any biologic drug or “biosimilar” is difficult enough, but especially in light of the most critical components of any drug, that it be both safe and effective. (Click to read: GaBi article on Small Molecule versus Biologic Drugs, and Amgen’s description of biologics and complexities in manufacturing biosimilars.) Plant-based biopharmaceuticals may well be an exception, since some products such as Moss-FH may have lower production costs (see the aHUS Alliance interview with Dr Ralf Reski on Moss-FH). In 2018 we’ve seen some pipeline expansion related to biosimilars, with three drugs specified in pharmaceutical pipelines as being under development as a biosimilar to eculizumab: Amgen’s ABP 959, ISU Abxis’ ISU305, and Genentech/ROCHE’s SKY59/RO711268. Success in developing biosimilars that match the safety and effectiveness of the original drug holds potential to reduce costs overall for both patients and the healthcare system, so the aHUS Alliance wonders whether new biosimilars might expand treatment options in nations where eculizumab is not currently available. While several pharma have expanded their pipelines with “extended release” drugs that extend time periods between administration of the drug, dosing intervals and new delivery methods seem important factors in 2018 regarding novel collaborative efforts at the corporate level. What remains to be seen is whether there’s a cost advantage in longer intervals and fewer treatment days for patients, the scope and degree of such inherent advantages, or if high drug discovery or production costs will result in negligible savings for patients, insurers, and others.
Limited drug access. The problematic topic of drug access has continued to spread and the aHUS Alliance anticipates that it will continue to do so for much of 2018, as drug access issues currently seem under-recognized regarding its impact on drug discovery and clinical trials in the aHUS arena. Almost worse than no available treatment for a specific rare disease population is recognition by physicians and patients that a drug is available, but access is limited or restricted. According to the Alexion Pharmaceuticals corporate site, more than 40 nations provide eculizumab availability for aHUS patients. The actual number of nations on Earth varies by definition, but for comparison purposes there are 206 Olympic nations, 195 participants in the United Nations, 211 countries eligible for the FIFA World Cup in football (soccer), and 249 country codes recognized by ISO standards. Comparing the value of a therapeutic drug with regard to its desired medical effect, patient benefits, allocation of healthcare resources, and other considerations (pharmacoeconomics) points toward need for data-driven study as part of the pharmaceutical research and development process. Patients currently lack ways to reconcile advancements in knowledge and drug discovery with the realities of access and clinical trials, so shifting paradigms of industry outreach toward more meaningful communication avenues may help pharma better understand the experiences of physicians and patients. This past year has seen an increase in social media stories and posts about struggles of aHUS families which are related to drug cost and drug access, as well as more online petitions to government regulators and Healthcare Ministers. In 2018 there’s also been an uptick in aHUS Alliance contacts by advocacy groups and physicians in multiple nations expressing general concern about drug access issues and increased interest in new therapeutic drugs. Given such medical and ethical considerations, one would assume that market research and drug discovery would include early input from physicians and patients about barriers currently faced in aHUS diagnosis and management. That has rarely been the case. Instead the aHUS Alliance reports that proactive outreach, meaningful dialogue, and coordinated flow of information remains largely an unanswered but important goal for aHUS stakeholders in the coming year.
Market conditions affect patient care. Similar to any market commodity, price and availability of orphan drugs will impact many aspects of atypical HUS patient care. Few corporate websites or investor press releases regarding biopharmaceutical advancements mention price or availability in relationship to drug discovery innovation, although logic implies that patients and institutional review boards (IRBs) would be among the key stakeholders to readily welcome safe and effective drugs with a small economic footprint. No matter how wonderful an orphan drug might be in regard to its ability to save or transform patient lives, what value does it retain in parts of the world where market conditions or high cost make the drug unavailable to physicians and their patients? In their 2017 article Orphan drug policies and use in pediatric nephrology by Karpman and Höglund2 included eculizumab as one of three drugs mentioned in their article noting, “Government interventions and regulations may opt to withhold a life-saving drug solely due to its high price and cost-effectiveness. Processes related to drug pricing, reimbursement, and thereby availability, vary among countries, thus having implications for medical care.” In Access to Orphan Drugs: A Comprehensive Review of Legislations, Regulations and Policies in 35 Countries, a review of the literature found that “Six broad categories of regulation and policy instruments were identified: national orphan drug policies, orphan drug designation, marketing authorization, incentives, marketing exclusivity, and pricing and reimbursement. The availability of orphan drugs depends on individual country’s legislation and regulations including national orphan drug policies, orphan drug designation, marketing authorization, marketing exclusivity and incentives such as tax credits to ensure research, development and marketing.” While Patient-Focused Drug Development (PFDD) was launched by the United States FDA with an intent to include patient views and input at very early stages of development, it also was meant to help identify and utilize approaches and practices regarding clinical trial enrollment and participation. Similarly the aHUS Alliance advocates for development of atypical HUS patient and caregiver panels, familiar with broad themes which affect aHUS families in all nations, as part of every industry or research effort. Some of the individuals best positioned to explain practicalities regarding the intricate interface of price, drug access, and healthcare policies are the physicians and clinical staff who treat people living with aHUS. Rather like the proverbial elephant in the room which is obvious, but no one is comfortable discussing, drug access and cost dramatically impacts the ability of physicians treat and manage aHUS patient care. Where eculizumab is not currently available, patients and physicians need to better understand forces in play as they advocate for expanded access and toward more treatment options. Pharmaceutical companies with investigational drugs have strong incentive to engage early with physicians and their patients. Better understanding of global concerns and treatment realities is especially critical for pharma whose drug R & D undergo progress from preclinical to clinical trials for patients with atypical HUS. Patient focused drug development holds value, and industry knowledge of physician concerns is vital, but central to dialogue and engagement is the need for better information flow to connect people, efforts, and opportunities.
Our review of the 2018 landscape for new therapeutics options for treating patients with the rare disease atypical HUS would be incomplete without examining issues and underlying factors driving research, drug discovery, advocacy issues, and market conditions. Following our two tables, the aHUS Alliance provides additional context to deepen the discussion regarding topics related to aHUS drug discovery and market conditions. This arena is evolving rapidly so the aHUS Alliance encourages others to create aHUS-specific articles and resources that offer a variety of information and assets, to suggest methods to keep current with growth and news in the aHUS space, to include international aHUS patient voices and physician viewpoints, and to provide insights that respect the dedication and acknowledge the valuable contributions of clinicians and aHUS research teams the world over.
Click to download the PDF of this 2018 aHUS Drug Discovery table.
Links to corporate websites or online assets are provided for your convenience and information, but note that content on such external sites may change or be removed and is outside the control or responsibility of the aHUS Alliance. While a curated overview, information in Table 1 should not be considered as fully comprehensive, and inclusion therein does not constitute endorsement by the aHUS Alliance.
Click to download the PDF of Diseases/Disorders with related research.
Fragmented information flow: A core problem
Finding accurate and updated information about any rare disease can be difficult. Why does the aHUS Alliance identify fragmented information as a significant problem that affects everyone and everything, to include drug discovery? Divergent or scattered information leads to missed opportunities to connect and maximize information flow, to lack of visibility for collaborative initiatives, and to the neglect of potential avenues which might advance efforts and knowledge. As research teams and industry have become drawn toward an incentivized orphan drug space, one would think stakeholders would become increasingly interested in robust and informed dialogue that supports development of new initiatives. More interested, yes – but increasingly the aHUS Alliance finds that even for-profit stakeholders seem to be missing sectors of background details or lacking connections among existing areas that could greatly impact their efforts. We find this a puzzling contradiction. Interface with international atypical HUS advocacy and global patient voices are available across a network of multiple platforms and formats, but who is listening to these valuable insights? What happens when third-party survey efforts in the aHUS space neglect to include clinical subtypes (e.g. long-term dialysis, newly diagnosed, patients with multi-organ involvement, transplant patients, and others)? How accurate can any forecast be when surveys or market reports center on data pulled mostly from nations where eculizumab is available, but which neglect to include information, views and concerns regarding nations where policy or cost limit drug access?
No matter what the service, product, or platform, an exchange of information and ideas is central to all aspects from concept to delivery. Bringing a new therapeutic drug to niche markets is admittedly a unique situation, but basic economic and market realities hold true. The aHUS Alliance is aware that industry must deal with regulatory and healthcare differences around the world, with policies3 for orphan drugs which can vary greatly from nation to nation. Professional organizations with business or research interests usually start initiatives with a thorough search of existing material and understanding of the market conditions to provide a basis for their team’s scope and sequence. The aHUS Alliance has experienced situations when a person or group reached out prior to their review of background information among nations with aHUS advocacy efforts, their social media streams, or material within global aHUS website or its aHUS Info Center. Two viewpoints are essential to begin an informed understanding of the aHUS space, that of patients and physicians. The best place to find out about aHUS patient experiences and concerns in a particular nation is to connect directly with advocates in that nation, and the aHUS Alliance has created a global directory of national aHUS advocacy groups to encourage those connections. Physicians who treat those with rare diseases can provide unique insights into atypical HUS diagnosis, management, and patient care issues so in 2017 the aHUS Alliance created a networking page for aHUS Clinicians and Investigators. Clinicians in various nations additionally can offer views about barriers within their national regulatory and institutional environments for patient care or research, assist with networking among medical professionals in their region, and help explore various challenges and possible solutions.
There are many challenges faced by rare disease patients that hold true within the aHUS community. Fragmented information flow impacts all areas, creating yet another barrier for patients but also for their physicians as well. In the case of aHUS, key research and information is often found under the category of complement-mediated disease but what about new knowledge which transcends current terminology or groupings? As noted in Nature Genetics by Lemaire et al in 2013, “DGKE is the first gene implicated in aHUS that is not an integral component of the complement cascade”. Should one assume physicians and patients might think that complement inhibitors may not be effective for that clinical sub-type? Switching specialties to ASH and C Quinn’s 2015 article in The Hematologist, “Complement-directed therapy with eculizumab may not be effective for patients with isolated DGKE mutations. However, this possibility should not prevent or delay empiric therapy with eculizumab when a clinical diagnosis of aHUS is first made, because genetic diagnosis takes time, and some patients with DGKE-associated aHUS may have secondary complement dysregulation.” Many people living with atypical HUS have a nephrologist as their primary care lead, so would that physician be aware of this information or about aHUS genetic testing in general? What implications for diagnosis and treatment come to mind with that phrase ‘secondary complement dysregulation’? Physicians also are faced with lack of consensus on several important topics, as noted within the KDIGO aHUS and C3G guidelines and emphasized by the use of the term ‘controversies conference’ within its title. It should not be surprising that adult aHUS patients and parents of pediatric patients are among those who have questions regarding duration of eculizumab therapy, some as a result of discussion with their medical team and others after reviewing research or news stories (such as our interview with Dr. G Ardissino). The aHUS Alliance will continue to provide information and updates on a variety of related topics, to include efforts underway in France (NCT02574403: STOPECU) and the Netherlands (CUREiHUS) regarding evaluating the duration of eculizumab therapy and following the precision medicine theme of ‘the right drug for the right patient at and for the right time’.
Connections Add Value
Given the small patient population for any rare disease and the difficulty in getting a new drug through regulatory, medical review, and clinical trial stages, we’re puzzled as to why information available in the public domain is often difficult to find with a resulting lack of context which makes information harder to interpret. Assets are scattered, key terms are not consistent, and a more accurate or holistic view takes time-consuming clicks across the internet and deep into sub-pages of multiple websites. A research paper in a hematology journal might lead the aHUS Alliance to a biotech stock column, which in turn might lead us to a press release on the company’s web page targeted to investors. We’d next search the corporate pipeline, which may provide the one or two specifics needed for us to find a related research article to uncover the desired factual information. What impact might a more streamlined information flow have for physicians or patient groups? What options exist for connecting information in the public domain, and could certain strategies have an impact on research grants, partnership opportunities, market analysis, or investor interest?
Creating and maintaining an information flow that’s comprehensive and accurate would seem a primary interest for companies. One would think that pharmaceutical companies would gather public information into an easily accessed and frequently updated ‘info packet’ or corporate sub-page about their drug discovery and clinical trial process. It’s not uncommon to stumble across a presentation slidedeck, conference poster, or corporate video that’s very relevant or provides a more holisitic view, yet it remains a resource which may not even mentioned or directly linked. There’s room for improvement on multiple fronts, as illustrated by these few examples. One pharmaceutical company separates its aHUS and PNH divisions, although the investigational drug is the same for both branches. Given the history of eculizumab’s initial start as a drug explored for use with PNH then its rapid consideration and FDA approval as a drug for patients with atypical HUS, the aHUS community has interest in what’s happening for advancement in PNH research and clinical trials. When different corporate branches and different teams are assigned the same drug and functions, but their efforts are artificially divided according to the rare disease community served, this seems a fragmented approach given the reasonable expectation of that large intersection of common needs and issues. Finding the time to volunteer as an aHUS advocate can be challenging when balancing work and family with medical treatments, and advocates appreciate when industry asks our participation while recognizing our time lost from work or offering options to allow aHUS advocates to have a voice at meetings. Building relationships and common background is integral to any business model, so it’s difficult when corporate restructuring or replacement of patient liaisons must begin anew. Why aren’t pharmaceutical companies and other industry stakeholders compiling key resources to provide a robust, curated resource to provide an accurate and updated asset that includes their own information available to the public? Our search results Alexion’s ALXN1210 found a link for Ravulizumab (ALXN1210) but Alexion’s corporate site lists recruiting clinical trials as Champion aHUS-311 and -312 while ClinicalTrials.gov returns other information. Athough ALXN5500 was listed along with ALXN1210 within an 2015 Alexion press release with both noted as next-gen versions of eculizumab, the aHUS Alliance found no further information connected with designation. Imagine how disjointed information must look for clinicians and other medical personnel. Most physicians will never see a single case of atypical HUS in their entire medical career due to its rarity, but some clinical trials list inclusion criteria (characteristics for eligibility) that notes a requirement of treatment naïve patients (no previous treatment). Given that key search terms are varied, information is scattered, and diagnosis of aHUS is so complex, can we reconcile such disparities and do they in part account for difficulty with aHUS clinical trial enrollment?
Engaging aHUS Patients in Research
While everyone agrees in theory that there are major benefits to be derived from a broad and connected flow of information, the aHUS Alliance sees multiple pathways that either remain to be built or are currently underutilized. There’s no one ‘right way’ to proceed even in a small, niche rare disease arena such as atypical HUS. What might encourage greater patient participation in research studies or clinical trials?
Core needs may seem obvious to some, but what is the perception and what is the actual reality? Adult aHUS patients and caregivers of pediatric patients have clearly stated their interest in becoming involved in atypical HUS research. The aHUS Alliance 2016 aHUS Global Poll included multiple questions to frame patient and caregivers’ views, and 233 respondents from 23 nations responded to survey questions. Section 5 of this 2016 aHUS poll was specific to patient engagement in research, with question 29 asking about participation in aHUS research. While 50% of international aHUS patients and caregivers gave a positive reply (answering either ‘Yes’ or ‘Yes, and would again’) it was highly interesting that about 36% of poll respondents chose “No, but I would like to learn more about how to be included.” There’s currently a disconnect between people and groups seeking patient engagement in the aHUS community, and their target pool of aHUS patients and caregivers of pediatric patients. What barriers currently exist, and what actions or initiatives might bridge them?
What would encourage greater patient participation in research studies or clinical trials? Question 34 of the aHUS Alliance 2016 aHUS Global Poll asked that very question, with the results below. Simply put, it’s apparent that outreach to patients and their physicians need to be reframed in order to become more effective. The top three responses all center on aspects best addressed by examination of information and communication flow.
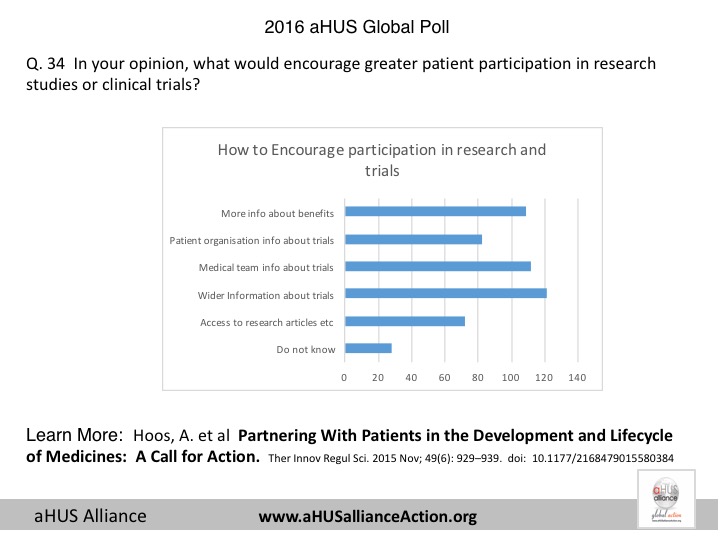
Survey Question 34 Outreach & Recruitment for Patient Engagement: What might encourage greater patient participation in research studies or clinical trials? (Source: aHUS Alliance, 2016 global poll, multiple response question, N=233)
52% – Wider offering of opportunities to receive information and updates about ongoing studies or trials
48% – More information from my (our) medical team regarding current or ongoing studies or trials
47% – Inform patients how their participation can benefit their own care, help other patients and/or their own family members
While patient engagement is merely one thread in the fabric of the 2016 global aHUS survey, it does distill the concept that patients and caregivers want to participate in research and efforts that might lead to better aHUS patient care options. Given the atypical HUS community desire to engage, where might patient engagement prove to be of value? Everything about patients should include patient input – according to a wide variety of individuals, groups, and articles within industry, research, and other professional journals. Some groups clearly value patient partnership, such as The BMJ which requests that authors co-produce educational articles with patients and caregivers. Among stakeholders within the aHUS space, who defines patient engagement, and how many opportunities exist for patient partnerships that actually engage and involve patients early and often?
Why are we including this in an aHUS Alliance article about investigational aHUS therapeutic drugs on the horizon in 2018? Atypical HUS patients, caregivers, and advocacy groups were contacted multiple times in 2017 but this usually occurred as an ‘add on’ effort after the stakeholders plans and initiatives had already launched. Even this late contact the value of aHUS community proved valuable, resulting in actions that included retooled surveys and reframed recruitment efforts. Of concern to the aHUS Alliance were a few third-party vendors who, when presented with input regarding specifics of aHUS patient needs or key concerns, opted to retain their pre-set study methodology or outreach avenues since “we’ve already started” or “we have x years of experience doing this same thing for other rare disease groups”. Patient engagement can add efficiency and help mediate the inherent risks in bringing a new therapeutic drug to market. In their 2015 article Partnering With Patients in the Development and Lifecycle of Medicines: A Call for Action Hoos et al wrote, “Drug development times are around 10 to 15 years and costs to bring a single new therapy to market are substantial. From the industry perspective, not putting the unmet medical needs of patients first, early in the development process, can lead to wrong priorities, wrong decisions on research design, and potentially costly late-stage failure.”
In a guest column for the Clinical Leader, Dr. Dominic Galante commented about slow progress regarding patient engagement in the pharmaceutical industry, “Developing treatments that can truly help improve patients’ lives should be rooted in a firm understanding of the daily challenges patients face, their needs, and the trade-offs they are willing to make to gain relief. To create valuable treatments, patient needs must be aligned in all aspects of the healthcare system, including research priorities, product development, trial design, regulatory approval, access, reimbursement, and treatment decisions.” He continued on to note the differences in “Despite this paradigm shift toward incorporating patient perspectives, pharmaceutical companies have yet to maximally engage with patients to learn what they value before developing a product. Most pharmaceutical companies have advocacy and market research groups that have networks and skills in patient outreach. These departments, however, tend to focus more on the commercial aspects of launch and post-launch activities, such as disease awareness and education. Individuals working in these areas often have different objectives for patient engagement than their colleagues in clinical development.”
Moving Forward: Identifying Issues and Opportunities for Stakeholders
Drug discovery can be advanced more rapidly when all involved share views and experiences to build a common understanding of context and conditions, issues and concerns, and connections which may point toward effective paths offering opportunities for progress. Bears in mind that only 20 years have passed since Warwicker, Goodship et al published Genetic studies into inherited and sporadic hemolytic uremic syndrome in Kidney International, an article which became pivotal in understanding aHUS as a complement-mediated TMA. (Click here to learn more.) Only about 5% of rare disease populations have even a single therapeutic treatment or approved product, and only about half of an estimated 7000 rare diseases have a patient advocacy group (Stein S et al, 2018). While aHUS patients are fortunate to have both an effective drug and disease-specific patient organizations, they still face issues common to all challenged by a rare disease. As noted in the 2017 article by Rodriguez-Monguio et al, “Rare disease patients and their families encounter health and financial barriers in the availability of diagnostic, prevention and treatment alternatives, coverage for existing care, general awareness in society and the healthcare system about rare diseases, and access to educational resources for their own comprehension of their condition.”4
International Advocacy. There’s a complex set of factors involved with development of any new therapeutic drug. Physicians and patient organizations in each nation are well suited to describe their nation’s healthcare system and rare disease policies, providing important nation-specific details about treatment concerns and patient needs. In the atypical HUS community for example, aHUS Canada navigates a very complex advocacy field since Canadian rare disease policies and drug access differ among provinces rather than adhere to nation-wide guidelines. (See article by Coles V et al 2017) There are important issues in the aHUS arena common to multiple nations, so connecting with global groups and efforts is vital. Clinical trials and aHUS research must have an international interface, and both drug discovery and patient treatment issues call for a global overview. Working with aHUS advocates and patient groups in nations all over the world, the aHUS Alliance provides a platform to share research advancements as well as broad-based insight and wide scope regarding common needs and topics that range across organizations and geographic boundaries. Patient organizations additionally interact with physicians and industry. Regarding rare disease advocacy and interactions with biopharmaceutical companies Stein et al5 note that patient advocacy organizations enable drug development and access by functions that include: “Educating patients, physicians, and the community about a disease and innovations in its management and treatment” and also “Providing drug developers with relevant insights into the patient community to enable the development of therapies that best meet the community’s needs”. Both of these aspects were key goals in creation of this aHUS Alliance article.
Multiple medical & research pathways. It will be difficult to move forward with drug discovery and treatment advances when aHUS terminology is confusing. With targeted therapy has come new challenges, to include the non-specific nature of the term aHUS which Nester and Smith (2014) note “At best, this term encompasses a group of diseases that share in common the clinical features of a microangiopathic hemolytic anemia associated with thrombocytopenia and renal failure. At its worst, however, aHUS lumps together a group of diseases with very different underlying pathologies. In practice, there is little agreement on what defines or limits classifying someone as an aHUS patient.” (See 2017 KDIGO Guidelines for aHUS and C3G, Kidney International) Collaborative research in atypical HUS and its genetics are spanning continents and broadening a shared knowledge base, as evidenced by the complement database, and research into its genetic variants such as a recent Journal of Immunology publication (Osborne et al, 2018). Physicians and aHUS patient organizations have seen publications that address an intersection of genetics and complement therapeutics from various perspectives, such as anti-complement factor H antibody (Kumar M et al, 2015), complement activation patterns during acute phase and remission (Volokhina EB et al 2015), and incomplete inhibition by eculizumab regarding residual C5 activity during strong complement activation (Harder M et al, 2017). Patient surveillance guidelines are a need for all phases aHUS activity and duration, and we anticipate new research into biomarkers (measurable indicators about the body’s health and specific conditions) will help monitor complement inhibition to add this important facet to existing knowledge. (See Riedl M 2016 and Wehling C et al 2017.) Information and research relative to atypical HUS may be tagged with search terms related to TMA, complement, or appear in research findings for diseases in fields such as autoimmune, inflammation, or rare renal genetics. Given this, the next generation of complement therapeutics is likely to see PNH, aHUS, and perhaps C3G as initial targets (Kavanagh D and Wong, 2018). Communicating clinical trial data and research findings must consider a variety of perspectives but everyone from investors to physicians and patient groups all need a better information flow to explain certain common elements that tie together scientific advancements with impact improving patient outcomes.
Varied Clinical Presentations. People with aHUS can have entirely different patient profiles, presenting with few outward symptoms but at risk for serious, sudden medical events. Atypical HUS can manifest with vague symptoms that share characteristics of other diseases, which may result in aHUS patients receiving a delayed or incorrect diagnosis for similar patterns of injury (differentiating among syndromes of TMA, or thrombotic microangiopathy). Patients with aHUS patients often present with wide differences in symptoms, degree of severity, and duration (acute or chronic) of active aHUS episodes. Atypical HUS is a systemic disease with 20% of patients experiencing impact on organs other than the kidneys, known as extra-renal manifestations (Loirat and Frémeaux-Bacchi, 2011). Any organ or body system functions may be damaged (TMA extra-renal manifestations, Hofer et al 2014) and such multi-organ involvement furthers compounds diagnosis and treatment of patients with suspected atypical HUS. This unpredictability, coupled with the rarity of aHUS, make diagnosis difficult and treatment complex. In 2018 there’s been somewhat of a paradigm shift toward more agreement of need for a multi-disciplinary approach to patient care (see Gordon CE et al, 2017) and also supported within an aHUS Alliance article on collaborative care. Recently we’ve seeing more research and journal articles on the topic of TMA differentiation, to include a recent publication of aHUS in the ICU (Azoulay E et al, 2017 ). The aHUS Alliance has created articles on this topic targeted toward patients and their families, and also collaborated with clinicians and researchers to create the MedEd event Thrombotic Microangiopathy Symposium: Through the Lens of aHUS (TMA Boston). Clinical trials and patient registries have had their difficulties in 2017, and may continue to do so as the global aHUS Patient Registry may face change in 2018. Integrating patient experiences and insights at all stages of initiatives and research provides opportunity to dig deeper while building a common background and solid footing to launch forward research and collaborative efforts (see aHUS Alliance Article on Patient Engagement).
Physicians as Key Partners. On the front lines of aHUS, physicians stand by their patients with expertise and a strong commitment toward improving patient outcomes. It’s hard to strike balance among economic realities, patient privacy, data silos, proprietary information, and a myriad of other factors. Physicians can provide valuable insight into systemic challenges for healthcare and treatment issues, but also into personal experiences with drug access and compassionate use situations that affect their patients and practice. Rare disease centers for excellence currently are not well defined, although much needed as the aHUS Alliance receives contacts requesting information related to aHUS expert care. In 2017 the aHUS Alliance started development of a network of clinicians and aHUS investigators who act as connecting points to advance aHUS research in practice, allowing insight into global and regional challenges faced by physicians or their patients. Models for physician education and engagement in the aHUS space would benefit from growth and support by all stakeholders. Outreach and information usually includes the fields of nephrology and hematology, but as previously mentioned more expansion is still needed across and throughout multiple disciplines.
Reframing Adult vs Pediatric Outreach. Both patients and physicians have strong interest in safe and effective treatment options that are widely available, but realistic factors of access to information and treatment are sometimes more difficult to determine and connect in meaningful ways to foster change. Most people understand the very valid reasons why clinical trials involve adult rather than pediatric patients at the outset, although patients may be less fully informed regarding other aspects such as factors involved to determine clinical trial locations. It’s time to recognize that physicians who treat pediatric patients largely remain an untapped wealth of information. Pediatric specialists are held to the same healthcare and institutional policies and management controls, and work within the same academic or hospital settings. They attend the same conferences and continuing education offerings as physicians who treat adult patients, networking and consulting with peers. (See our article on collaborations at IPNA Bucharest). Today’s pediatric patients with chronic disease are tomorrow’s adult patient population. Physicians working with pediatric patients can share views about needs for parent/caregivers and for issues involved with transitioning their young patients to adult care. Physicians who treat adult aHUS patients have a specialized vantage point that allows them to convey what their patients face, such as the need for adult patients to self-advocate on treatment issues, and patient pressures that are social, economic, or work-related. Informal consultation and networking among physicians happens regularly, so we encourage a broader view for those seeking physician engagement and insight within the aHUS arena. Physicians are among the stakeholders experiencing frustration related to the economic and ethical dilemmas created by limited treatment options and drug access, so we suggest fostering relationships and channels to gain physician input and to acknowledge the value of their message by taking actionable steps.
aHUS patient interest in research. There’s a complex set of factors involved with development of any new therapeutic drug. While most look at the ‘front end’ of drug research and development at the industry end, it’s important not to lose sight of issues involved with bringing an investigational drug to market and what ‘end use’ concerns might exist for patients and those who treat them. The aHUS Alliance and other advocacy groups continue to reach out to all stakeholders, but actively seeking and offering information and ideas is quite different than early and regular inclusion of patient perspectives as valued participants within a broad and meaningful network. This lack of continuity and flow in the information stream will continue to hinder progress, until bridges link what currently exists and pathways are proactively built to connect stakeholders, information, and opportunities. (See the aHUS Alliance 4 part series on this topic). Fragmented information is a barrier to research and clinical trials. Given that key search terms are varied, information is scattered, and diagnosis of aHUS is so complex, can we reconcile such disparities and do they in part account for difficulty with aHUS clinical trial enrollment? There are varied restrictions on public advertisements for approved drugs around the world, but among disclaimers is often the phrase “Ask your doctor about (the product)”. According to question 38 of the 2016 aHUS Alliance Global Poll (N=233, from 23 nations), 37% of aHUS patients and caregivers for pediatric patients turn to aHUS patient organizations as their key information source while only 17% rely on the physician for information about their disease. Information can ‘trickle up’, with 20% of survey participants additionally stating that they spend enough time and effort discovering aHUS-related content to allow them to share aHUS information with their medical team. What might this indicate about effective avenues for patient outreach, and need to build new pathways to exchange ideas and information?
What’s behind that survey, report, or group? No matter what the topic it’s always important to view information, resources, and initiatives with knowledge and background that provides an array of meaningful facts and insight gathered from multiple sources. The same is true in the aHUS arena, for all stakeholders. Look at the websites and social media for atypical HUS advocates and patient organizations to determine their interests, goals, international collaborations, original content and resources as well as their efforts regarding aHUS issues, needs and concerns. Are broad issues and international aspects included? What are their accomplishments and concerns? Look at aHUS market analyses, presentations, and reports to determine study methodology, references cited, survey population, inclusion of international issues, reference to varied market conditions around the world, and depth of background information. What evidence is present to indicate a knowledge of conditions and issues within the aHUS arena? Are the challenges and perspectives of physicians included, and if so how and to what degree? Is the patient voice integrated rather than tacked on as an afterthought, with patients seen as valued partners? (See aHUS Alliance article on this topic) Look at aHUS research publications, centers of study and research, consensus documents, and education initiatives efforts to ascertain people and groups involved and to further explore their focus, interface with international colleagues or professional affiliations, and impact within the aHUS arena. As noted by Bunnik et al, 2017 in their article about ethical implications regarding investigational drugs and patients with unmet medical needs. “Physicians, patients and policy-makers should not shift the responsibility to address these issues to pharmaceutical companies, but work together to resolve them.” The aHUS Alliance believes that it is everyone’s responsibility to address issues of drug access and other key issues, and that all stakeholders (to include pharmaceutical companies) should be included in dialogue and comprehensive solutions within partnerships dedicated to building a brighter future for aHUS patients and their families.
Explore global issues, insights, and advocacy. The aHUS Alliance launched its first international aHUS Awareness Day in 2015 and we continue to raise awareness with our annual 24 September campaign on Twitter @aHUS24Sept, with year round atypical HUS advocacy Tweets and information @aHUSallianceAct. Launched in 2016 the aHUS Alliance website now has topped over 50,000 views of its original material, designed to highlight aHUS content and written by an international team of volunteers who themselves are aHUS patients or caregivers. Overviews of topics and needs within the atypical HUS community have been created, such as global Interim aHUS Patients’ Agenda. Since there are aHUS patients in nations all over the world, the aHUS Alliance has developed networking pages to speed those connections to national advocacy groups and to aHUS clinicians and investigators. We’ve utilized inclusive and innovative approaches to connect information and present insights into aHUS issues and needs around the world. The aHUS Alliance conducted a 2016 aHUS Global Poll (233 respondents from 23 nations) to help provide data that national aHUS patient organizations could utilize for policy meetings with their Healthcare Ministry, but which additionally provides viewpoints and perspectives of the aHUS community on topics such as inequity in drug access and treatment options. The aHUS Alliance partnered with the scientific advisory board of the aHUS global registry to produce a 2016 Orphanet article, which included international patient engagement in research priorities and paths forward for collaborative endeavors between research teams and aHUS patient advocacy groups. (Woodward et al, see the related aHUS Alliance article ). Patient experiences framed TMA clinical presentations at the previously mentioned TMA Symposium ‘MedEd’ event held at the Joseph B Martin Center at Harvard Medical School, with patient advocates participating from three countries (Canada, UK, USA: see the TMA symposium playlist of videos). In these examples, international atypical HUS advocacy issues were integrated throughout to offer a deeper dive into the experiences of aHUS patients and caregivers in terms of diagnosis, management, treatment issues, and lifestyle impact.
By offering a global aHUS advocacy perspective of drug discovery that includes market factors affecting patients and physicians, this aHUS Alliance article connects varied information to provide a simple 2018 snapshot of potential new aHUS therapeutic drugs. An authentic patient voice is central to all aspects of healthcare, policy, and research. We’re the survey respondents and the subjects in clinical trials, we interact with dedicated physicians and navigate across the full spectrum of healthcare. Who better than a knowledgeable aHUS advocate to explain gamut of patient experiences, from the frustration and despair over limitations in treatment options to the celebration of each successful medical intervention toward better health? It’s time to consider patients and aHUS advocacy groups as partners who can provide unique insights and detailed opinions that can productively speed research or product development along a more efficient path by helping others better target and tailor their efforts.
L Burke, May 2018
About the aHUS Alliance
As an international group of aHUS patient organizations, the aHUS Alliance reaches out to provide news and insights into atypical HUS research and key topics of high interest to the global aHUS community through website, Facebook, and Twitter content. Specific groups and nations involved in aHUS advocacy around the world can be viewed in our listing of known patient associations or outlets with an aHUS website or social media presence.
The aHUS Alliance website at www.aHUSallianceAction.org is operated on behalf of the aHUS Alliance by aHUS Alliance Global Action, registered as a charitable incorporated organization in England and Wales, Registration No. 1167904.
Contact Us: info@ahusallianceaction.org
Citations
- Loirat C and Frémeaux-Bacchi V. Orphanet J Rare Dis 2011; 6: 60. doi: 10.1186/1750-1172-6-60 (Section: Disease Name and Synonyms)
- Karpman D and Höglund P Orphan drug policies and use in pediatric nephrology. Pediatr Nephrol. 2017 Jan;32(1):1-6
- Dharssi S, Wong-Rieger D, Harold M, and Terry S. Review of 11 national policies for rare diseases in the context of key patient needs. Orphanet Journal of Rare Diseases 201712:63 doi.org/10.1186/s13023-017-0618-0
- Rodriguez-Monguio R et al. Ethical imperatives of timely access to orphan drugs: is possible to reconcile economic incentives and patients’ health needs? Orphanet J Rare Dis. 2017; 12: 1. doi: 10.1186/s13023-016-0551-7.
- Stein S et al. Principles for interactions with biopharmaceutical companies: the development of guidelines for patient advocacy organizations in the field of rare diseases. Orphanet J Rare Dis. 2018; 13: 18. doi: 10.1186/s13023-018-0761-2
Resources regarding New Therapeutics and
Complement Drug Discovery
2018
Ricklin D et al. The renaissance of complement therapeutics Nat Rev Nephrol. 2018 Jan; 14(1): 26–47.
Ristano A and Marotta S. Toward complement inhibition 2.0: next generation anticomplement agents for paroxysmal nocturnal hemoglobinuria. Am J Hematol. 2018;93(4):564-577. doi: 10.1002/ajh.2501
Harris Claire L. Expanding horizons in complement drug discovery: challenges and emerging strategies. Semin Immunopathol. 2018; 40(1): 125–140.
Additional Publications
Antwi-Baffour S et al. The relative merits of therapies being developed to tackle inappropriate (‘self’-directed) complement activation. Auto Immun Highlights. 2016 Dec; 7(1): 6.
Joost PM et al. Complement in therapy and disease: Regulating the complement system with antibody-based therapeutics. Molecular Immunology. Volume 67, Issue 2, Part A, October 2015, Pages 117–130
Morgan, B. Paul and Harris, Claire L. Complement, a target for therapy in inflammatory and degenerative diseases. Nature Reviews: Drug Discovery. Nature Reviews Drug Discovery: 14, 857–877 (2015) doi:10.1038/nrd4657. Published online: 23 October 2015.
Reis E et al. Applying complement therapeutics to rare diseases. Clin Immunol. 2015 Dec; 161(2): 225–240. doi: 10.1016/j.clim.2015.08.009
Ricklin D and Lambris J. Complement in Immune and Inflammatory Disorders: Therapeutic Interventions. doi: 10.4049/jimmunol.1203200The Journal of Immunology April 15, 2013vol. 190 no. 8 3839-3847
Waters A and Licht C. aHUS caused by complement dysregulation: new therapies on the horizon. Pediatr Nephrol. 2011 Jan; 26(1): 41–57. doi: 10.1007/s00467-010-1556-4

E: info@aHUSallianceAction.org Atypical HUS – Global Rare Disease Advocacy
