The reluctant advocate’s journey continues, ( click here for the story so far).
Our NICE evidence was submitted on 9 September 2013. The day after that it was the second anniversary of the forming of aHUSUK. That day we heard that the interim aHUS service recommended by CPAG had been ratified by the NHS Board. It would be three months until the next NICE meeting.
Time for a rest. Not so.
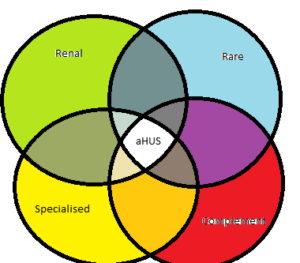
Two years after its creation aHUSUK was very active in the communities relevant to aHUS – Renal- Rare/Genetic -Complement- Specialised Health Services. The reports on aHUSUK’s website in that period reflect that.
-The charity was invited by the Bio Industries Association or BIA for a “breakfast meeting” in the House of Parliament with politicians, pharmaceutical industry and health organisation representatives. We were, uniquely, able to comment on an experience of going through two evaluation processes for a treatment for our illness AGNSS and NICE. It was interesting to hear the industry’s side of matters and their commitment to and investment in research; and one statistic was very surprising. Of the €70 billion invested in research into developing medicines for rare diseases, €60 billion is invested by the US government and Pharmaceutical Companies in almost equal measures and a creditable €5 billion by academia/patient based charities. Leaving just €5 billion spend by the governments and others in the rest of the world. If true rare disease patients depend a lot on the USA. Makes you think.
-We attended the first public meeting of a new major project for cancer and rare diseases. ..The 100,000 Genomes Project. Its aim was to undertake full genome testing of 100,000 patients in England. This was being done to find possible genetic causes of their diseases. aHUS was just one such diseases that had a genetic cause. However not all genetic reasons had been discovered. Nearly 40% of those tested were found to have no known genetic mutation. The meeting was held in the Great Hall of St Barts Hospital London. Its walls were covered with 18th century murals painted by William Hogarth, another historic and impressive building visited as a patient advocate. aHUSUK was fully supportive of this project even though aHUS patients would be a very small number of potential participants.
– aHUSUK had been asked to join the Rare Disease Group for aHUS. This initiative was led by the UK renal professional body “The Renal Association “ and funded by the major renal charities like Kidney Research UK. The group’s task was to set guidelines for the diagnosis and treatment of aHUS . It also provided encouragement for research into the disease. It was chaired by Professor Tim Goodship.
– We also attended the annual meeting of Complement UK , an organisation set up to develop knowledge and understanding of the Complement system and it impact on diseases such as aHUS. That meeting was an eye opener in so many ways not just from the presentation on aHUS but also how different mutations in different components of Complement led to it having too little or too much activity resulting in a spectrum of diseases. Complement plays a part on its own or in conjunction with other parts of the immune system in diseases such Altzheimers , Multiple Sclerosis , Parkinson’s , Antiphospholipid Syndrome, Age Macular Degeneration, as well as renal diseases like Lupus , MPGN and aHUS. Complementology could be an important specialism in the future!
– Soon after the Complement UK Meeting aHUSUK attended a meeting about all Renal Rare Disease Groups in Peterborough to develop a cross group understanding and support. Inspired by one of the patient representatives there, that meeting led to a aHUSUK research fund raising project. But that was one for the future.
– aHUSUK thoughts were also turning to the next Rare Disease Day, in particular to a project to create an artwork to be revealed on that day , The “Raise Your Hand” was seeking the names of 2000 patients to be a “named hand ” and aHUSUK was encouraging as many aHUS patients and carers as possible to join in and show support.
– aHUSUK had given evidence to the Scottish Government about how health technologies for cancers and rare diseases were evaluated. The work of the Scottish Medicines Consortium , a sort of NICE for Scotland, had been studied by a Scottish Government Committee which had been set up following petitioning by the Rare Disease Community. The vast majority of drugs for rare diseases had been turned down by SMC unlike those for common diseases. That Committee had submitted its report. The Scottish Government was consulting stakeholders on its proposal for change. aHUSUK provided its views.
– As was the case in England and Scotland NHS Wales was also re-evaluatiing its processes for highly specialuised technologies undertaken by the Welsh Strategic Health Committee. aHUSUK were invited to attend a focus group meeting to provide feedback to the task group which would be reporting its recommendations to the Welsh Government. It did begin to seem more than coincidence that all UK health authorities ( Northern Ireland would follow what was happening to NICE) were suspending , reviewing and changing their process just as Eculizumab for aHUS had entered or was about to enter evaluation.
-Genetic Alliance UK were working with the NHS and NICE to design a “Patient’s Charter for the appraisal of rare disease treatments” As aHUSUK had experiences of AGNSS and uniquely NICE too we could contribute a lot to the discussion, although with great care as we were in the midst of the NICE process.
-three trustees attended the Annual General meeting and Conference of the National Kidney Federation and stood a table in the exhibition area to raise awareness about aHUS and its kidney patients.
-Of course the charity itself had to be administered and the third general meeting was held in Solihull in the centre of England to make travel more equidistant for those in all regions. Apart from the administrative part of the day , including the “Treasurers Report” the role I had actually been volunteered for , part of the day was given over to a conference about aHUS . Prof Goodship continued to give his support with up dates about developments in aHUS. The conference was also addressed by Phyllis Talbot a director of the USA’s not for profit patient group, The Foundation for Children with atypical HUS. She did it while driving with her family from her home in Atlanta to Baltimore for Thanksgiving and it was via Skype, projected on to a large screen. A technical feat only spoiled by a hitch when the connection was lost at the end and before she could see the standing ovation she received. aHUS was a small world and aHUSUK’s outreach to other countries patient groups was growing.
All this plus more being reported on our website and in a very active social media, and also with regular newsletters being sent to members. More patients were contacting us and needed suppport and assurance as they their families faced an encounter with aHUS for the first time.
Yep there was much to do still and this period illustrates how running a charity made more and more demands on its volunteers and their time. Three new Trustees had joined us and then two of them resigned, such was the burden of commitment and workload needed.
Why would anyone be reluctant to do all that?
Keeping plates spinning just while we were at it.
It being the main aHUSUK objective to get eculizumab approved free for all patients when they needed it for as long as they needed it..
Three months passed quickly and soon it would be the 11th December 2013

