So adverse events are being reported. In USA over 4500 per year on average for eculizumab are reported to the FDA and just over 400 per year for ravulizumab. But the public information cannot be used to draw conclusions about risks and safety.
Adverse events and patient reported outcomes feedback is an important way to both understand the long term nature of aHUS and what happens when a patient is treated. There are probably 10,000, or more, patients who have received eculizumab or ravulizumab by now. It has been used to treat a range of diseases, but most prominently used for PNH and aHUS. Some patients now may be approaching 20 years of being treated, I.e those who were in early PNH trials.
After the drugs were licensed by the FDA , EMA , etc Alexion has been obligated to report on the drugs continued safety. “Continued” because the drugs safety was first assessed in clinical trials. During those trials the most serious adverse event of meningococcal infection emerged. In 2019 the first 10 year safety report i.e. up to 2016 was published. It was a joint PNH/ aHUS report. The conclusion of that report was that there was little difference to what was found in the trials.
That did not mean there were no more adverse events, but just that little new events had been found through long term use. But patients were still experiencing somethings possibly from using the drugs..
How did Alexion know that ? It was because there is continuous minitoring system in place in which patients and their clinician can report adverse events.
For patients in USA and Canada with a OneSource case manager have that as a route to let Alexion to know about adverse events they have experienced. For others, the countries contact details are included on the prescription leaflet which comes with each vial of eculizumab/ravulizumab. See image below.
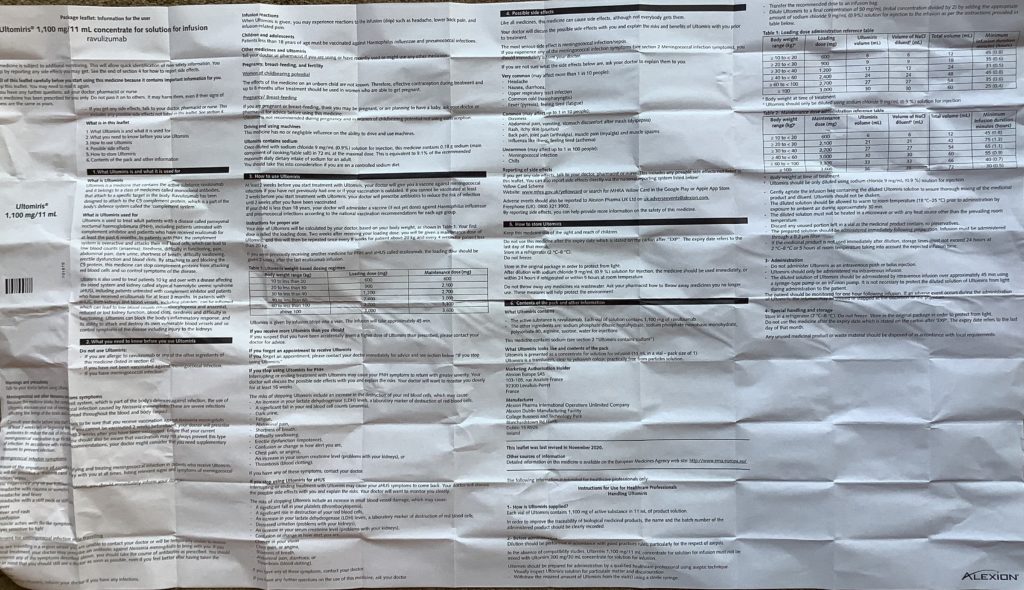
In the example above the contact details are included at the end of Section 4 – Side effects ( see a copy of Section 4 below for the UK contact details). The section ends with the statement ” By reporting side effects you can help provide more information on the safety of the medicine. “
That is a call for MORE information. That can be new side effects which may be not known to Alexion or more examples of side effects already known about, but whose incidence is under reported and understated.
What is already known about is listed on the prescription leaflet ( see image below) They are listed in the order of the chance of PNH / aHUS patients experiencing them, very common , common or uncommon. Out of 10,000 or more patients who have used eculizumab / ravulizumab 1000 or more of them are likely to experience very common symptoms like headaches, less than 1,000 of the 10, 000 patients will experience common symptoms like itchy skin and no more than 100 out of 10, 000 will experience uncommon ones like meningococcal infection . Looking at it another way if the 10,000 patient are on treatment for a year then the chances of a patient having meningococcal infection is once in every 100 years.

Note :The MHRA is the UK equivalent of the FDA or EMA.
How many aHUS patients know about this and has anyone experienced something different to the side effects listed. how many aHUS patients have reported their adverse events? If a side effect happens every time should it be reported every time? How patient friendly is what they have to do to report a side effect.
So those discussing mutual symptoms on social media have an outlet that can capture their experiences.
And Alexion would have a truer picture of the incidence of adverse events and it would be something that they would in turn need to report.
But remember Debbie’s point made in the RDD2022 Video about helping the patient with those problems. Does “very common’ make the symptom less or more of interest to do something about it. And how would we know if something is being done?
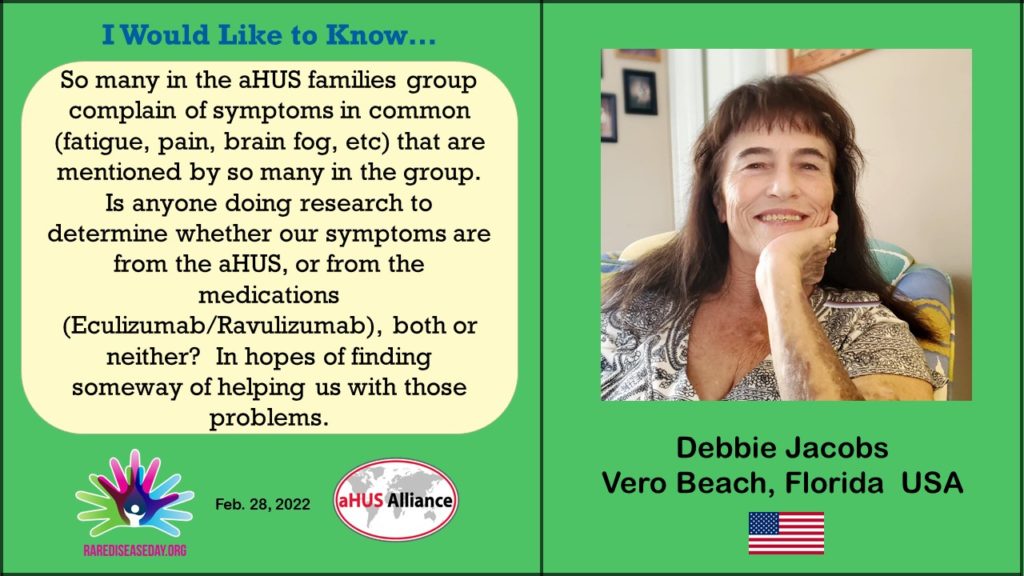
If all patients reported all their side effects what would result. Would the truer picture alter the risks and benefits of using eculizumab / ravulizumab? Probably not. Would it create an incentive for the research that Debbie asks for? The jury is out.
Would it help patients to know that they have provided more information and added to aHUS awareness? Almost certainly. But it is down to individual patients whether they will , or prefer to just tell their peers on social media.
Would it help if there was a generic e mail address which anyone on eculizumab and ravulizumab could use , anywhere in the world where Alexion market those drugs. Maybe. And there is such an e mail. It is given below
AdverseEventReporting@alexion.com
And what about patient reported outcomes. Well that is another matter.
Article No. 555

