Article No. 354
4 July 2020
Earlier this year aHUS Global Action published a major four part report (see HERE) on the importance of patient reported outcome data in aHUS care research. The report ends with a case study of the utility of such data for aHUS fatigue in the Alexion aHUS Registry.
This website has often featured articles about the fatigue felt by aHUS patients. It is a symptom and effect of aHUS that matters to them.
A recent news article on our website (see HERE ) revealed that when it comes to an “aHUS Fatigue” search on the internet, aHUS Global Action articles appear foremost within search engine results. This illustrates our contribution to awareness about the topic.
If the word “publication” is added to the search term, Global Action articles remain prominent but now an article appears which was published in May 2020 in the Kidney International Reports journal of the International Society of Nephrologists.
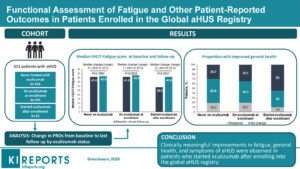
“Functional Assessment of Fatigue and Other Patient-Reported Outcomes in Patients Enrolled in the Global aHUS Registry” is the result of a study which Global Action has been anticipating for three years ( first reported in this website in a 2017 article HERE)
The KIR article is free to view and can be read HERE.
It is very well written and easy to understand but with substantial statistical/numerical content.
Using data from patients’ reported outcomes to the Alexion Registry, the article merely describes what they have to say. Well, over 500 of them that is ,as the data from the other 1300 or so enrolled patients‘ data was unusable, or a decision was made to not use it. e.g. any data from those over 65 yers old were excluded, as too were patients’ reports from those who had withdrawn from eculizumab treatment.
The 551 patients ( 23% children and 60% female) included in the Study were divided into three groups.
Firstly those who been enrolled after they had already started Eculizumab treatment ( 295) Then there were those who commenced eculizumab treatment after they had been enrolled (23) and finally there were those who had never been treated with eculizumab (233).
As previously referred to a large and important cohort who had withdrawn from eculizumab treatment sadly were excluded from this study so it is not known what happened to them. Neither is the experience of the “late on-setters ”.
Of those included and who potentially could have provided data, only the minority of each group had actually recorded useful data. 43% of those treated prior to enrollment , 19% of those treated after enrolment and 43% of those never treated with eculizumab .
The shortfall of data is not unusual in Patient Registries, The hopes and expectations on creating one tend to be dashed in the practicalities of running one. In total 59% of potential patient reported data was not available to be used.
In this study the focus was to be on the smallest cohort from the Registry , those who began eculizumab treatment after being enrolled with sufficient data reported i,e, 23 patients (Including 3 children).
This was the group where the timeline from being ill and untreated to getting eculizumab and recovering from the aHUS episode can be fully described. Albeit this group had the shortest follow up time line, 18 months on average compared with 24 months (on ecu before enrollment) and 30 months (never on eculizumab).
Using the Function Assessment of Chronic Illness Therapy Fatigue scale – “FACIT- Fatigue” the study group found aHUS patients in this category showed the greatest improvement in feelings of fatigue from their pre treatment baseline. The conclusion being that eculizumab apart from stopping uncontrolled and damaging complement activity, leads to better overall quality of health.
Their overall median fatigue scores at their illness low point was 32, ( computed from 29 for children and 34 for adults), but with treatment, for on average 18 months it became 41 at midpoint An extremely significant improvement of 9 points , given that a three point score change alone would be regarded as significant for adults.
Additionally as a fatigue score for the general population would be around 44 those on eculizumab treatment can be said to be returning towards “normal” but at that moment they still had significantly higher tiredness symptoms, which would need more time to recovery.
As this is an ongoing longitudinal study we will have to wait to see what happened to them with further follow up.
As for the other two groups although data did not show the vast improvement that the “treated after enrollment“ cohort experienced. Though the study revealed that they too were approaching normal and were remaining steady at that level whether they ever have received eculizumab or not. The mid point for those never on eculizumab being 42 and those already receiving eculizumab being 43.
It is worth emphasising that these are mid point scores and that a minority of patients reported an increase in fatigue from baseline. Equally some reported much lower levels of tiredness.
There needs to more understanding of when symptom becomes an effect. The study does not address what causes the fatigue and whether physical ( e.g. haemoglobin) and psychological (e.g. PTSD) factors , or both, are at play. Neither is the impact of any other interventions mentioned.
Improving beyond “normal“ is within an individual aHUS patient’s grasp.
For those current aHUS patients just starting treatment with Eculizumab/ Ravulizumab it could be of some comfort to know that things can return to normal. Patience is needed.
There is more to this study than observations about aHUS fatigue and which warrant further examination in another Global Action blog to follow.
But for now the aHUS patient community has yet another answer on a topic which matters to them.
In that regard there is one reference in the introduction to this study that is worthy of highlighting.
The authors refer to “A Global aHUS Patients’ Research Agenda using input from patients, patient advocacy groups, and caregivers was developed.9 The research agenda identified several knowledge gaps, including the impact of aHUS-related treatments on clinical, psychological, and socioeconomic patient-reported outcomes.“
The agenda that aHUS patients helped create to express what matters to aHUS patients ( more about it HERE) is cited as the reason for undertaking this research. This is something to be applauded.
This specific research study is describing iwhat aHUS patients themselves are saying about something which has been asked for by aHUS patients themselves because it matters to aHUS patient.
What better purpose for aHUS research can there be?
Hopefully, in future, other researchers will chose to hang their aHUS research studies hat on the aHUS patients’ own research agenda.
Featured image is of the graphical abstract from the KIR article