In its 11 December 2018 press release, the Omeros Corporation announced a new collaboration with a UK university to create the Omeros Center at Cambridge for Complement and Inflammation Research (OC3IR). Focus of the OC3IR will be research into OMS721 and OMS906, click HERE to read the corporate press release. “This partnership represents a tremendous opportunity to benefit from the renowned complement and inflammation expertise at Cambridge,” stated Gregory A. Demopulos, MD, chairman and CEO of Omeros whose press release remarks continue, “The result, we expect, will be good for Omeros, Cambridge and, most importantly, patients.”
Omeros’ drug development pipeline has been of interest within the aHUS arena for quite sometime, and has been mentioned in aHUS Alliance pharma overviews to include most recently our May 2018 aHUS Therapeutic Drug Pipeline edition. Atypical HUS is a very rare disease, affecting an estimated 2 people per million, with diagnosis made difficult by having common symptoms similar to other forms of thrombotic microangiopathy. This innovative center at Cambridge is not only a new opportunity for scientific and drug discovery stakeholders, but OC3IR brings an expanded horizon of pathways to engage in dialogue. It also broadens the depth and scope of people at Omeros and the University of Cambridge involved with the new center, causing aHUS advocates to ponder the following questions (which are not listed in any particular order).
- Renal diseases are therapeutic targets for Omeros, with complement research noted as a key function of the OC3IR. In 2017 the UK’s National Renal Complement Therapeutics Centre won the ‘Research Delivery Impact’ award for its aHUS program at the Bright Ideas in Health Conference. Will the National aHUS Service and other key programs in the aHUS, complement, and renal disease fields be included in some way?
- Will the Omeros Center at Cambridge for Complement and Inflammation Research reach out to an international array of experts in the field, or will their work and action plans be nation-specific to the UK?
- In July 2018 aHUS Alliance advocates visited 4 aHUS expert centers in 3 nations on its aHUS Whistle Stop Tour. In November 2018, several nations attended the international aHUS Patient Advocacy Symposium held in Nimegen, NL. Days later, ERKnet held the webinar ‘Unraveling the pathophysiology of HUS in light of recent discoveries on complement activation’ with Dr Marina Noris as presenter. Given this level of activity and interest, will the staff at OC3IR create an interface with this existing network of interested parties? If so, how and if not, why?
- Many nations do not have access to what is currently the only drug approved to treat aHUS, eculizumab. Policy makers often mention high cost as a reason why less than 50 of 200+ countries have access to eculizumb, which negatively impacts kidney transplant options for aHUS patients as well. Will part of the efforts at OC3IR focus on creating safe and effective drugs that are also affordable for patients around the world?
- Physicians treating aHUS patients often face difficult choices, and this frustration has led to a proposal for an international physicians panel for aHUS drug access. To what degree has Omeros reached out to physicians to gauge their needs, and what are such plans for the future?
- Small patient populations are always an issue for any clinical trials meant for a rare disease, but here are many more barriers to aHUS clinical trials. Patient and physician recruitment have been problematic for other pharma in the aHUS space, so how is Omeros utilizing that information and those experiences to craft a more effective and proactive plan for their clinical trials?
Physicians, research teams, and aHUS patients and their families are looking forward to seeing how the Omeros Center at Cambridge for Complement and Inflammation Research plans to create better patient outcomes for those challenged aHUS and other diseases.
More on Atypical HUS
aHUS Press Kit – Fact Sheets, Graphics, White Papers, aHUS Awareness Day (24 Sept campaigns) & More
Omeros – Links & Info
Omeros Corp: 1 Oct 2018 (press release)
“Omeros also has identified MASP-3 as responsible for the conversion of pro-factor D to factor D and as a critical activator of the human complement system’s alternative pathway”
“Phase 3 clinical programs are in progress forOMS721 in atypical hemolytic uremic syndrome (aHUS), in immunoglobulin A (IgA) nephropathy and in hematopoietic stem cell transplant-associated thrombotic microangiopathy (HSCT-TMA).”
Information from Omeros Corp. regarding OMS906 – Click HERE
Of the 327 clinical trials for thrombotic microangiopathy, here are a few on this topic:
NCT03205995: Safety and Efficacy Study of OMS721 in Patients With Atypical Hemolytic Uremic Syndrome (aHUS)
NCT02222545: Safety and Efficacy Study of OMS721 in Patients With Thrombotic Microangiopathies
NCT02355782: OMS721 Compassionate Use in Patients With Thrombotic Microangiopathy
From Omeros Pharmaceutical’s corporate website, Investors
Click HERE and scroll down to ‘Featured Presentations’ to view the fullpresentation. (Next-Generation Therapeutics TransformingPatient Care Today, presented by Gregory A. Demopulos MD, Omeros Corp. Chairman & CEO)
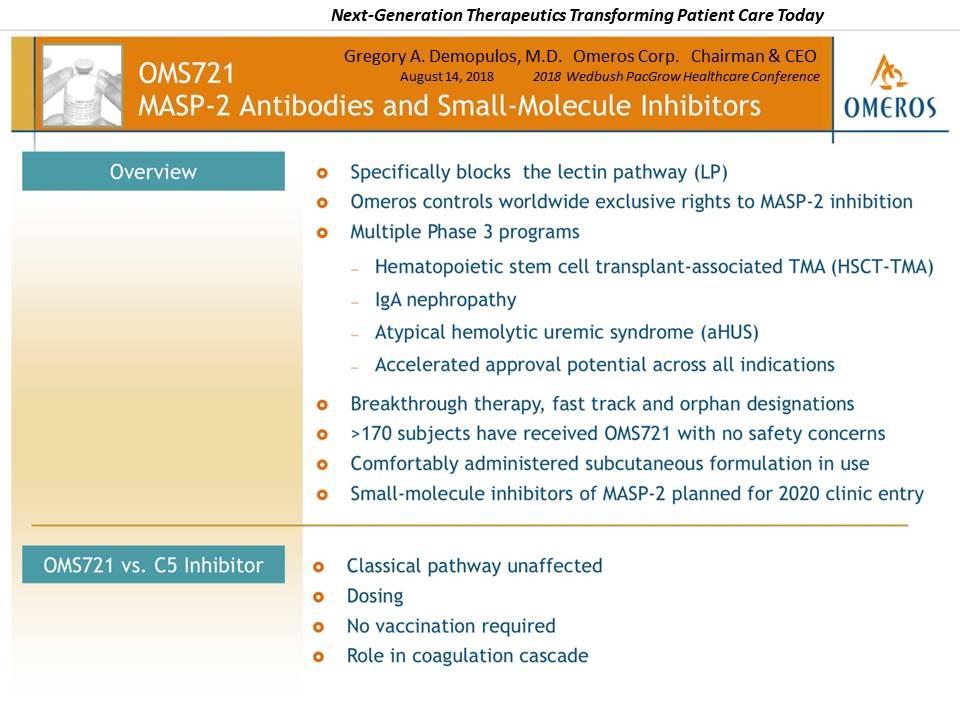
Presentation Slide #3: Omeros Pipeline
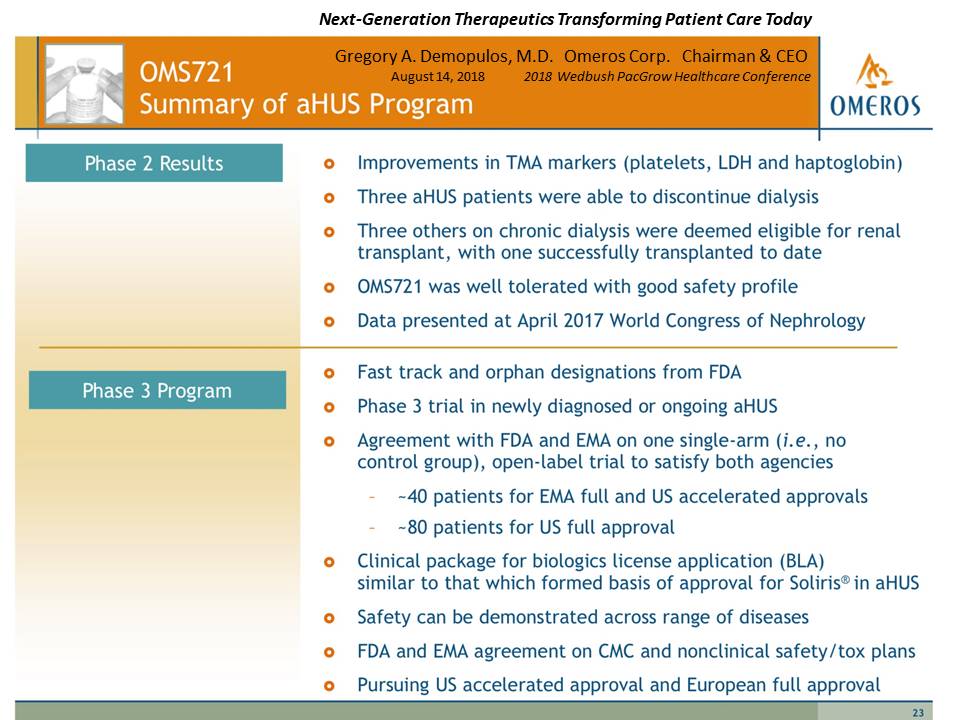
Presentation Slide #23: OMS721, Summary of aHUS Program
Presentation Slide #11: OMS721, MASP-2 vs C5 inhibitor