In the battlefield of the human body, atypical hemolytic uremic syndrome (aHUS) is a stark example of “friendly fire”—a term borrowed from military contexts where one side mistakenly attacks its own forces. In aHUS, the immune system, tasked with protecting the body, turns against its own tissues, causing devastating consequences.
The Battle Within: Self vs. Non-Self
The human body is in a constant state of war, with the immune system as its standing army, perpetually vigilant against “foreign invaders”—pathogens, toxins, or even transplanted organs and, in part, a developing fetus. Many of these invaders enter through routes, such as the mouth and nose, infiltrating the digestive or respiratory systems, and eventually reaching the blood and organs. The immune system’s mission is clear: identify and eliminate anything that is not “self.”
This immune army comprises different fighters. Some, like the innate immune system, are always on high alert, ready to engage at a moment’s notice. Others, part of the adaptive immune system, mobilize only when called upon, tailoring their response to specific threats. Among these defenders, the complement system—a key component of the innate immune response—stands out for its rapid and ruthless action.
The Complement System: A Lethal Weapon
The complement system is a group of proteins in the blood that work together to detect and destroy foreign invaders. When activated, it deploys a fearsome weapon: the Membrane Attack Complex (MAC). If scaled to human size, the MAC would be a terrifying force, piercing its target and causing it to “explode to bits.”
In the bloodstream, this weapon is a hero against invaders like the Group A streptococcal bacteria which causes “strep throat”, obliterating them with precision. Fire away, and the invader—our enemy—is defeated.
But this fire is not always friendly. When the complement system misfires, it can turn its destructive power on the body’s own tissues, particularly the delicate endothelial cells lining small blood vessels. In aHUS, this is precisely what happens: the complement system’s attack becomes indiscriminate, targeting healthy cells as if they were invaders. This is the essence of friendly fire in aHUS—an immune system gone rogue, attacking the very body it is meant to protect.
The Breakdown of Command and Control
In a healthy immune system, the complement’s destructive power is tightly regulated. Command-and-control mechanisms, involving proteins like complement factor H, ensure that the MAC targets only foreign invaders and shuts down rapidly to prevent collateral damage. In aHUS, however, this regulation fails. Genetic mutations in complement genes (e.g., CFH, CFI, C3) or autoantibodies that impair regulatory proteins disrupt this control, leaving the MAC unchecked. The result is uncontrolled friendly fire, with the complement system relentlessly attacking the body’s own endothelial cells.
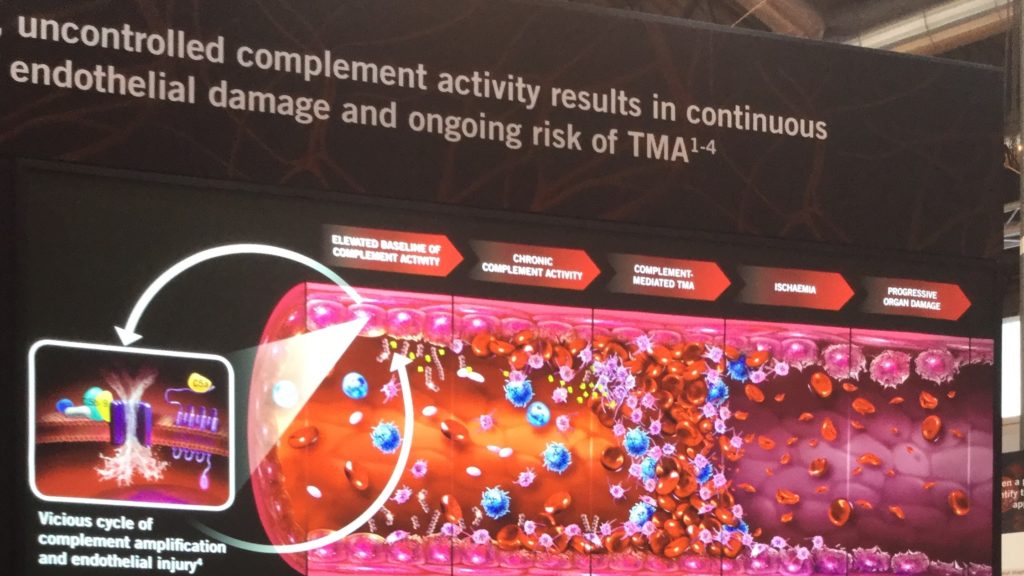
This misdirected assault causes widespread damage, particularly in the kidneys, where small blood vessels are abundant. The carnage triggers a cascade of secondary effects: red blood cells are destroyed (hemolytic anemia), platelets are consumed in excessive clotting (thrombocytopenia), and the kidneys suffer acute injury, often progressing to end-stage kidney disease (ESKD) if untreated. This triad of symptoms—hemolytic anemia, thrombocytopenia, and kidney damage—defines aHUS as a thrombotic microangiopathy (TMA).
The Aftermath: Clotting and Chaos
The friendly fire in aHUS sets off a chain reaction of destruction. As the MAC damages endothelial cells, the body responds by forming platelet-rich clots to repair the injured blood vessels. These clots, however, obstruct blood flow, leading to tissue ischemia and further organ damage.
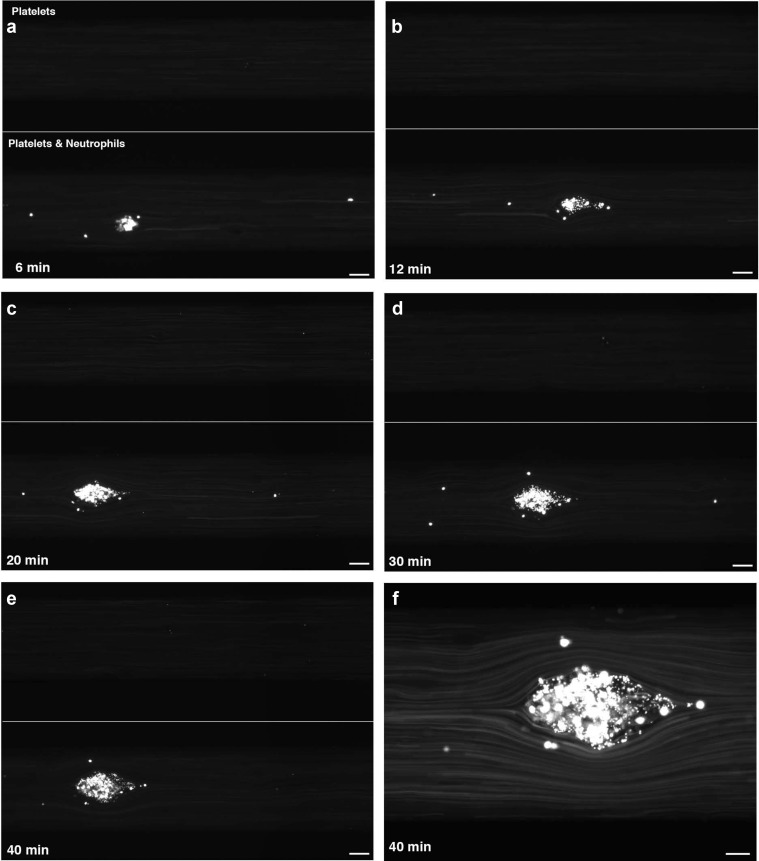
Excessive clotting is a feature of other disorders like thrombotic thrombocytopenic purpura (TTP) or typical HUS, what sets aHUS apart is its root cause: uncontrolled complement activation attacking the self. This relentless friendly fire distinguishes aHUS from other TMAs, making it a unique and challenging condition.
Article No. 744

