Ravulizumab is more widely used these days, particularly in the PNH community. Apart from the longer infusion intervals PNH patients benefit from higher dose levels that stop breakthrough haemolysis which, over time, more and more were experiencing with eculizumab
Side effects should be now be comparable with eculizumab too. It is the same active ingredient, and now is even infused at the same rate. Headaches and short term fatigue immediately following infusions were the most reported adverse events by users of eculizumab. Whether it was used to treat PNH or aHUS.

Ravulizumab leaflet insert
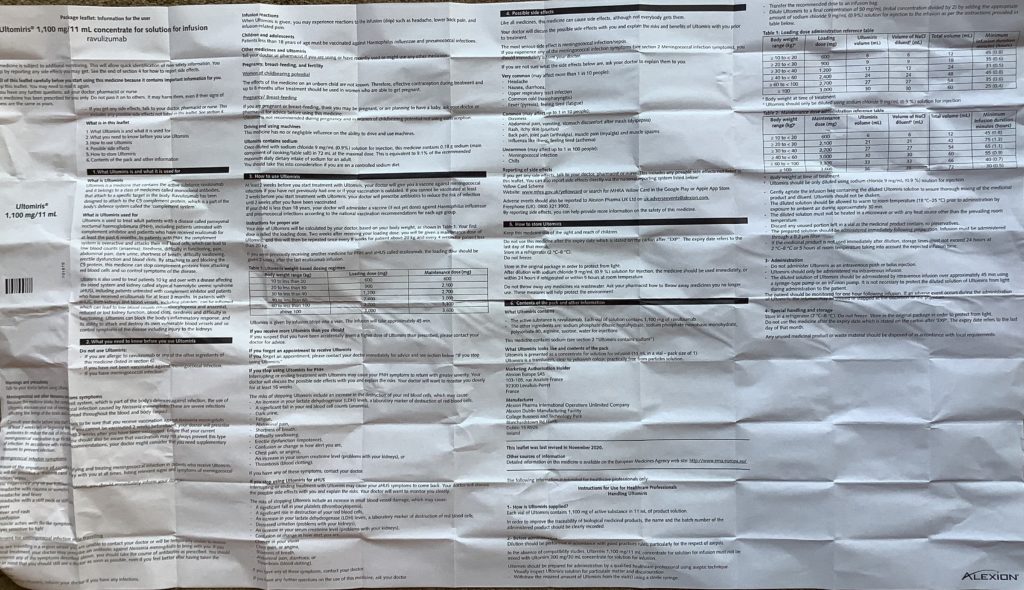
With every package of ravulizumab there is a leaflet inserted with Information for the user. Usually folded small ( see above image) to get into the vial package, but when spread out it is massive , about 25 inches by 15 inches ( see image below)
One of the first messages on the leaflet is ” this medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting side effects you may get. See the end of Section 4 for how to report side effects”
This would apply to you, as much as any one else Jennifer . Your experience is just as important.

Skipping to Section 4 , its title is “Possible side effects”. ( a copy of the section can be found at the end of this article). Alexion acknowledge there may be side effects, the most serious of which meningococcal infection /sepsis. Which is why patients are vaccinated for the common serotypes of the disease, and may also take prophylactic antibiotics.
Then there are lists of side effects which are very common ( may affect more than 1 in 10 people), common ( may effect up to 1 in 10 people and uncommon which may affect up to 1 in 100 people,
If by “very run down” you mean feeling tired Jennifer, that is a side effect listed in both the very common and common lists.
In the very common side effect list, feeling tired is also referred to as fatigue. As a common side effect is also referred to as asthenia.
The latter is more about feeling weak , lack of energy/strength. Fatigue is more feeling tired, wanting to sleep.
Fatigue is also a residual effect of an episode of aHUS. What is now being thought of as “long aHUS” .
But your symptoms only present late on in the infusion interval before the next is due. It has happened twice .That seems different.
The leaflet advice to users is to talk to you doctor, pharmacist or nurse about what concerns you. Whether it is explainable or not you can report what you feel to your nation’s adverse effects reporting system. Details of which for the USA are given in Section 4 of the USA leaflet.
Section 4 of the leaflet concludes by saying ” By reporting side effects you can help provide more information on the safety of this medicine”. Alexion has a team that will examine what it is reported, it may be something which they are already looking into .So your evidence would add weight.
It is up to you Jennifer. If you have not seen the leaflet in the ravulizumab vial packet ask the person performing the infusion for a copy to read.
A copy of Section 4 of the ravulzumab package leaflet follows:

Article No 511